
Semiconductor technology underpins comprehensive advancements in information and energy sectors, fundamentally transforming human production and lifestyles. The first-generation semiconductors, Germanium (Ge) and Silicon (Si), are the most maturely developed. The second-generation semiconductors are Gallium Arsenide (GaAs) and Indium Phosphide (InP). The third-generation semiconductors, Silicon Carbide (SiC) and Gallium Nitride (GaN), have produced and continue to produce profound and lasting impacts in power electronics and RF microwave fields.
In the periodic table, GaN belongs to the III-V group compounds. Depending on the type of chemical bonds, its crystal structure can be hexagonal wurtzite or cubic zinc blende. In compounds, two types of chemical bonds mainly exist: ionic and covalent. The higher the ionic bond content, the more likely it is to form a wurtzite structure. Due to the significant electronegativity difference between Ga and N, they are more likely to form ionic bonds. Therefore, at room temperature, GaN exhibits a thermally stable, corrosion-resistant wurtzite structure.
Compared to first and second-generation semiconductor materials, GaN has a wide bandgap, low dielectric constant, high critical field strength, high electron mobility, high thermal conductivity, high-temperature resistance, and excellent radiation resistance. Due to these excellent material properties, GaN shows tremendous potential in fast chargers, transistors, RF microwave devices, and more, making it a research hotspot globally. Table 1 shows a comparison of material parameters between GaN and other semiconductor materials. This article provides a detailed explanation of the preparation methods for GaN single crystal substrates.
1. Hydride Vapor Phase Epitaxy (HVPE)
HVPE, or Hydride Vapor Phase Epitaxy, features a fast growth rate and the ability to produce large crystals, making it one of the most mature technologies and the primary method for commercially available GaN single crystal substrates. In 1992, Detchprohm et al. first used the HVPE method to grow GaN films (400 nm), attracting widespread attention. While HVPE processes are mature and have a fast growth rate, they have drawbacks such as low crystal quality yield and poor product consistency. Typically, heteroepitaxial growth methods are used, wherein GaN is grown on sapphire or Si substrates and then separated to produce single crystal substrates using techniques like thermal decomposition, laser lift-off, or chemical etching.
2. Metal-Organic Chemical Vapor Deposition (MOCVD)
MOCVD, or Metal-Organic Chemical Vapor Deposition, has a stable growth rate and high-quality growth, suitable for large-scale production. It is one of the most mature and widely used technologies. MOCVD was first proposed by Mannacevit in the 1960s and matured in the 1980s. In MOCVD growth of GaN single crystals, trimethylgallium (TMGa) or triethylgallium (TEGa) are primarily used as gallium sources, with TMGa being the most commonly used due to its melting point. NH₃ is used as the reactive gas, and high-purity N₂ as the carrier gas. Under high temperatures (600~1300℃), thin layers of GaN are successfully deposited on sapphire substrates. Despite MOCVD's advantages of high product quality, short growth cycles, and high yield, it is expensive due to costly raw materials and requires precise control over the reaction process.
3. Ammonothermal Method
The Ammonothermal method is one of the primary methods for growing GaN today. First proposed in the 1990s, researchers were inspired by the hydrothermal method used for mass production of quartz. The growth process is similar to the hydrothermal method, but with ammonia replacing water as the solvent. As illustrated in Figure 3, by controlling the temperature, the autoclave can be divided into two zones: a dissolution zone and a growth zone. Due to the temperature gradient between these two zones, convection occurs. The process involves transporting polycrystalline GaN or Ga (the Ga source) to the dissolution zone to dissolve. Through convection, the dissolved Ga source is transported to the growth zone, where supersaturation conditions enable the crystallization and growth of GaN. The unreacted Ga source then returns to the dissolution zone due to convection, repeating the cycle to continuously obtain GaN.
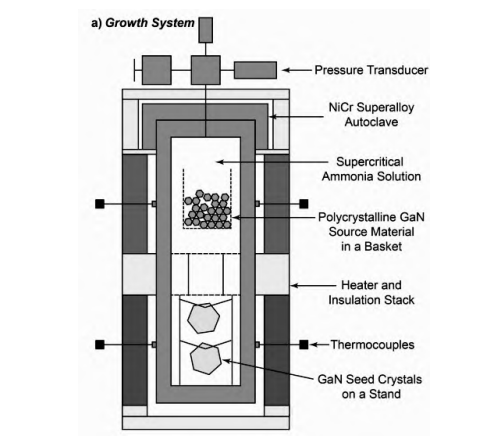
During the growth process, the solubility in the growth zone is insufficient to support continuous growth of GaN. To increase solubility and thus yield, mineralizers are added. Currently, common mineralizers are divided into two categories based on their acidity or alkalinity: acidic mineralizers for low temperatures, such as XNH2 (X = Li, Na, K), and basic mineralizers for high temperatures, such as NH4Y (Y = Cl, Br, I).
The Ammonothermal method produces GaN with high purity and high crystal quality but has drawbacks such as low growth rates and low yield.
4. Flux Method
The Flux Method is a representative method for growing single-crystal GaN and is an improvement on the High-Pressure Solution (HPNS) method. The HPNS method has slow growth rates and requires high-temperature, high-pressure conditions (GPa levels, temperatures above 1500℃). In the 1990s, Yamane first proposed adding Na to liquid Ga as a flux, known as the Na-Flux method. In the early 21st century, Yusuke Mori used the Na-Flux method to grow 2-inch GaN single crystals. The Na-Flux method reduces pressure from GPa to MPa levels and lowers the growth temperature to about 750℃. The growth process requires GaN single crystals grown by MOCVD as substrates for epitaxy. The principle of the Na-Flux method is that nitrogen gas dissociates into N atoms before entering the Ga-Na solution, which can stably exist in the solution. When the N solubility exceeds the critical growth concentration of GaN, it spontaneously nucleates for epitaxial growth. The Na-Flux method produces high-purity, high-quality GaN single crystals but requires high-quality equipment, has a low growth rate, and is mainly influenced by N₂ flow.
In addition to these methods, other techniques for preparing GaN single crystal thin films, such as Atomic Layer Deposition (ALD) and Magnetron Sputtering, exist. However, the crystal quality and purity of GaN films grown by these methods are low, making them unsuitable for commercial production.
Conclusion
Gallium Nitride (GaN), as a third-generation semiconductor material, holds great potential and broad application prospects in power electronics and RF microwave fields. Through various preparation techniques such as HVPE, MOCVD, Ammonothermal Method, and Flux Method, high-quality GaN single crystal substrates can be obtained. However, each method has its advantages and disadvantages, and the choice of preparation method depends on application needs and economic considerations. As technology continues to develop and improve, GaN single crystal preparation methods will become more efficient and economical, further promoting its application in various high-tech fields.
Related product links
Gallium Nitride Wafer (GaN Wafer)
As semiconductor technology advances, Gallium Nitride (GaN) has become a core material in optoelectronics, rad...
With the rapid advancement of third-generation semiconductor technologies, Silicon Carbide (SiC) has emerged a...
As third-generation semiconductor materials such as silicon carbide (SiC) and gallium nitride (GaN) continue t...
